CBS News Live
CBS News Los Angeles: Local News, Weather & More
Watch CBS News

Vector control specialists hope this will reduce the mosquito population without deploying pesticides and chemicals.

Caltrans said crews will repair the roadway starting at 9 a.m. April 29.

A civil case brought by an actress against Harvey Weinstein will be on temporary hold while the disgraced producer's criminal conviction for the same 2013 Beverly Hills attack against his accuser is on appeal, a judge ruled Thursday.

An assistant DA in George Gascón's office is facing felony charges over alleged misuse of police records.

The Los Angeles Chargers drafted Notre Dame offensive lineman Joe Alt with the fifth pick in the NFL Draft.

A woman in her 60's was shot and killed at an apartment complex in Anaheim Thursday.

Senior citizens in Little Tokyo are gathering each week for exercise and fun times at Terasaki Budokan, a multi-purpose sports and activities center.

Former National Enquirer boss David Pecker appeared on the stand for the third day, detailing an agreement the tabloid made with a former Playboy model.

The Oceanside Pier is nearly 140 years old.

A woman in her 60's was shot and killed at an apartment complex in Anaheim Thursday.

All of the late-season rain falling on Southern California has created the possibility of a bad mosquito season this year. However, vector control specialists have devised a way to reduce the population, even though it may seem counterproductive. Nicole Comstock reports.

Come down to the Eastmont Community Center between 10 a.m. and 4 p.m. on Saturday for the El Gran Festival of East LA.

Firefighters arrived about five minutes after the first call. Crews battled the flames for nearly three hours and contained them to the pier's hammerhead. They said the vacant restaurant and a neighboring brine box sustained significant damage. Lauren Pozen reports.

Harvey Weinstein's 2020 conviction on rape charges has been overturned by the State of New York Court of Appeals, which ordered a new trial. Weinstein will remain behind bars since he was convicted in a separate case in California.

The Denver Nuggets moved to the brink of the second round with a 112-105 victory over the Los Angeles Lakers in Game 3 of their first-round series.

The Los Angeles Chargers drafted Notre Dame offensive lineman Joe Alt with the fifth pick in the NFL Draft.

It's a decision that has been widely projected as a virtual lock for this NFL Draft, especially after the Bears traded away Justin Fields to the Steelers.

Reggie Bush said getting back the Heisman Trophy was like being on the field again.

Anze Kopitar fired a wrist shot past Stuart Skinner's glove and into the top corner on a breakaway to give the Los Angeles Kings a 5-4 victory over the Edmonton Oilers on Wednesday night in Game 2 of the first-round playoff series.

Luka Doncic scored 32 points and the Dallas Mavericks overcame the return of Clippers superstar Kawhi Leonard to beat Los Angeles 96-93 and tie their Western Conference first-round playoff series at a game apiece.

Mike Trout hit his first leadoff home run since 2012, and the Los Angeles Angels defeated the Baltimore Orioles 7-4 to snap a five-game losing streak.

Shohei Ohtani hit a 450-foot homer to the second deck in right field in his first visit to Nationals Park, and the Los Angeles Dodgers beat Washington 4-1.

Zach Hyman had three goals and an assist in his first postseason hat trick, Connor McDavid had five assists and the Edmonton Oilers beat the Los Angeles Kings 7-4 in Game 1 of their first-round playoff series.

How much crime is there in our schools? How safe are our airports? You have the right to know these things through public records but, as Ross Palombo has found, the state has some serious issues with what they are and are not telling us.

"No teacher should have to lie as part of their job". Ross Palombo talks to a teacher in the Los Angeles Unified School who says he was told to lie about class attendance, which is how the state calculates funding from your tax dollars.
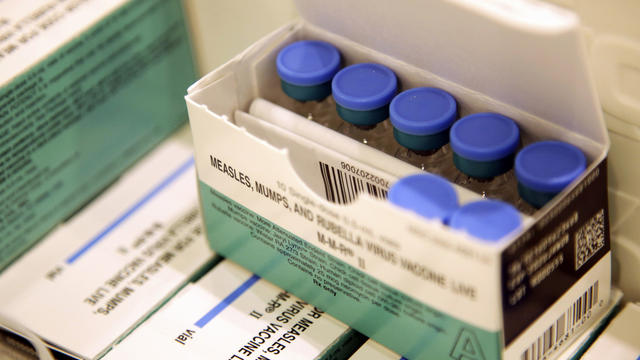
The CDC recommends that at least 95% of students have the MMR vaccine in order to lower the risk of measles outbreaks at their school, but a recent KCAL News investigation shows that hundreds of SoCal schools are well below that number.

For months, migrants have arrived in Los Angeles on controversial buses chartered from Texas. With a lack of coordination from the Lone Star state, community groups stepped in to help bring things under control and helped hundreds across Southern California.

Watts will raise awareness of serious issues impacting Californians, hold local officials accountable, obtain answers for viewers and provide solutions.

California law says genetic testing companies have to get your permission before they store, use or sell your DNA, but the state itself doesn't have to get your permission and has been storing DNA samples from every baby born there since the '80s. Lawmakers want to change that, but face an uphill battle.

A bill introduced in the wake of our "Handcuffs in Hallways" investigation aims to reduce "unnecessary" calls for police at schools. But one California lawmaker could kill it without a vote.

Vector control specialists hope this will reduce the mosquito population without deploying pesticides and chemicals.

Caltrans said crews will repair the roadway starting at 9 a.m. April 29.

A civil case brought by an actress against Harvey Weinstein will be on temporary hold while the disgraced producer's criminal conviction for the same 2013 Beverly Hills attack against his accuser is on appeal, a judge ruled Thursday.

An assistant DA in George Gascón's office is facing felony charges over alleged misuse of police records.

The Los Angeles Chargers drafted Notre Dame offensive lineman Joe Alt with the fifth pick in the NFL Draft.

A civil case brought by an actress against Harvey Weinstein will be on temporary hold while the disgraced producer's criminal conviction for the same 2013 Beverly Hills attack against his accuser is on appeal, a judge ruled Thursday.

Senior citizens in Little Tokyo are gathering each week for exercise and fun times at Terasaki Budokan, a multi-purpose sports and activities center.

The Los Angeles Police Department identified the homeless outreach officer who shot an armed suspect in Skid Row last week.

On Your Side's Kristine Lazar has the story of a 93-year-old whose caregiver allegedly stole thousands from her.

Summer will be here before you know it and we have your first look at the season's hottest beauty buys.

Police say that the incident began as a fight between roommates but escalated into a physical altercation early Sunday.
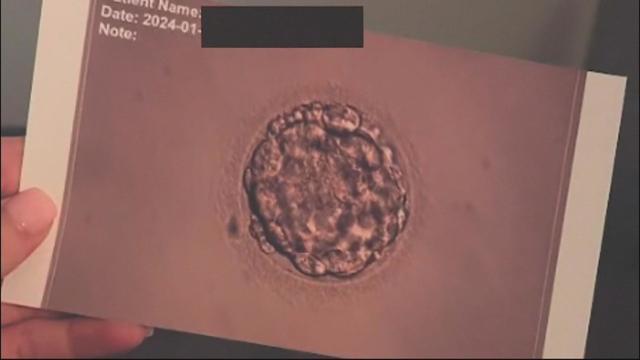
Nine Southern California couples have filed a lawsuit accusing the Ovation Fertility lab of negligence and recklessness.

Officers pulled over Jamie Rodgers after the dealership accidentally reported the car they loaned to him as stolen.

One person has died and two others were injured in a single-vehicle crash in Santa Ana.

The crash happened when a driver broadsided another car after failing to yield to right of way at the intersection of Placentia Avenue and Center Street, police said.

Vector control specialists hope this will reduce the mosquito population without deploying pesticides and chemicals.

A 21-year-old mother is luckily sitting in her home with her two children after she was ambushed and shot in the face outside an Apple Valley gas station last week.

The bust was part of a year-long operation dubbed "Hotline Bling."

San Bernardino County deputies arrested multiple people for trying to steal from a freight train at the Cajon Pass in what resembled a scene from an old western movie.

The Santa Clarita Valley Sherriff's Department received reports of a man with long hair shooting BB guns, and "windows have been shattered."

"No teacher should have to lie as part of their job". Ross Palombo talks to a teacher in the Los Angeles Unified School who says he was told to lie about class attendance, which is how the state calculates funding from your tax dollars.

The CDC recommends that at least 95% of students have the MMR vaccine in order to lower the risk of measles outbreaks at their school, but a recent KCAL News investigation shows that hundreds of SoCal schools are well below that number.

For months, migrants have arrived in Los Angeles on controversial buses chartered from Texas. With a lack of coordination from the Lone Star state, community groups stepped in to help bring things under control and helped hundreds across Southern California.

KCAL senior reporter Ross Palombo gets an unprecedented look into the tactics the LAPD uses to stop retail theft.

A federal court ruling involving a controversial arrest within Moreno Valley Unified School District could lead to big changes in school policies nationwide.

The Denver Nuggets moved to the brink of the second round with a 112-105 victory over the Los Angeles Lakers in Game 3 of their first-round series.

Former National Enquirer boss David Pecker appeared on the stand for the third day, detailing an agreement the tabloid made with a former Playboy model.

An estimated 53 million Americans are unpaid caregivers, many as part of the "sandwich generation" — simultaneously raising children and caring for aging parents.

Harvey Weinstein's 2020 conviction on felony sex crime charges has been overturned by the State of New York Court of Appeals.

As Israel's leader equates U.S. university protests to rallies in Nazi Germany, Palestinian students tell CBS News what the support means to them.

Former National Enquirer boss David Pecker appeared on the stand for the third day, detailing an agreement the tabloid made with a former Playboy model.

Gavin Newsom was asked to comment about Harvey Weinstein's 2020 rape conviction being overturned on Thursday, and the California governor didn't mince words.

There are no cameras allowed in the court where Trump is being tried on 34 felony counts stemming from a "hush money" payment before the 2016 election.

Regulators prohibit new noncompetes, which impede millions of U.S. workers from getting a better job.

The Senate passed the foreign aid package, which includes a provision that could lead to a ban on TikTok, after months of disagreement in Congress.

Target, looking for ways to add sales, is relaunching its Target Circle loyalty program including a new paid membership with unlimited free same-day delivery in as little as an hour for orders over $35.

When, who and how much to tip is becoming more of a question in consumers' minds.

One in four college students says they have credit card debt, according to a new survey by U.S. News and World Report. And with interest rates topping 20 percent, those students could be in debt for years. On Your Side's Kristine Lazar has more on the biggest mistakes college students are making with their credit cards.

Fewer than a third of Americans have a will. Experts say that could lead to confusion and money loss when someone dies without one. On Your Side's Kristine Lazar has expert tips on who needs a will and how to create one.

Choosing random numbers increases your chances of not having to split the prize money should you win.

California law says genetic testing companies have to get your permission before they store, use or sell your DNA, but the state itself doesn't have to get your permission and has been storing DNA samples from every baby born there since the '80s. Lawmakers want to change that, but face an uphill battle.

Fentanyl test strips used to be illegal in California. Now, state law requires them on community and state college campuses and they're popping up everywhere from vending machines to bars. They're intended to help young people avoid fentanyl-laced counterfeit prescription pills and tainted recreational drugs. But as fentanyl test strips are normalized – from high school to college to bachelor parties – experts warn test strips alone can provide a false sense of security, and in some cases do more harm than good. We put fentanyl test strips to the test, and what we found could save someone you know.

Dr. Gary Gibbon is now cancer-free, six months after the first-ever lung-liver transplant on a cancer patient.

Preventative chemotherapy, which is usually referred to as "adjuvant chemotherapy," is an early treatment that is used to reduce the chances of cancer returning.
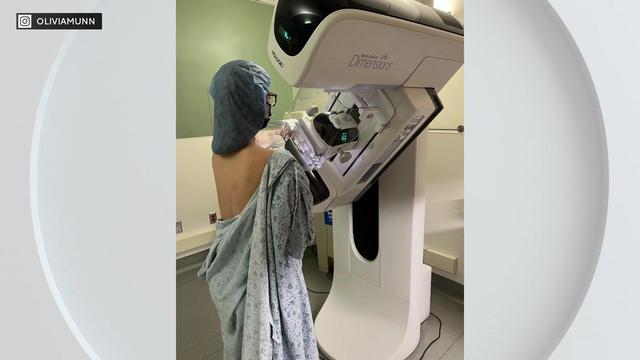
Actress Olivia Munn's cancer diagnosis is raising awareness for women to seek additional screening for breast cancer after her traditional testing failed to show any signs.

Robotaxis are hitting the streets of Los Angeles.

Disney is seeking approval from local officials to expand its California theme park offerings over the next four decades.

Target, looking for ways to add sales, is relaunching its Target Circle loyalty program including a new paid membership with unlimited free same-day delivery in as little as an hour for orders over $35.

Southern California Edison will pay $80 million to settle claims on behalf of the U.S. Forest Service connected to a massive wildfire that destroyed more than a thousand homes and other structures in 2017.

As he closes in on the Republican presidential nomination, former President Donald Trump made a highly unusual stop.

The Denver Nuggets moved to the brink of the second round with a 112-105 victory over the Los Angeles Lakers in Game 3 of their first-round series.

The Los Angeles Chargers drafted Notre Dame offensive lineman Joe Alt with the fifth pick in the NFL Draft.

It's a decision that has been widely projected as a virtual lock for this NFL Draft, especially after the Bears traded away Justin Fields to the Steelers.

Reggie Bush said getting back the Heisman Trophy was like being on the field again.

Anze Kopitar fired a wrist shot past Stuart Skinner's glove and into the top corner on a breakaway to give the Los Angeles Kings a 5-4 victory over the Edmonton Oilers on Wednesday night in Game 2 of the first-round playoff series.

Harvey Weinstein's 2020 conviction on felony sex crime charges has been overturned by the State of New York Court of Appeals.

Taylor Swift fans have found a way to feel "a little bit closer to" their hero at a London watering hole, and The Black Dog pub is lapping it up.

The Spice Girls had a reunion on Saturday and even put on an impromptu performance.

Roman Gabriel, the former North Carolina State quarterback who was the 1969 NFL MVP with the Los Angeles Rams, has died.

The singer was found deceased at her home, a representative said.

A woman in her 60's was shot and killed at an apartment complex in Anaheim Thursday.

All of the late-season rain falling on Southern California has created the possibility of a bad mosquito season this year. However, vector control specialists have devised a way to reduce the population, even though it may seem counterproductive. Nicole Comstock reports.

Come down to the Eastmont Community Center between 10 a.m. and 4 p.m. on Saturday for the El Gran Festival of East LA.

Firefighters arrived about five minutes after the first call. Crews battled the flames for nearly three hours and contained them to the pier's hammerhead. They said the vacant restaurant and a neighboring brine box sustained significant damage. Lauren Pozen reports.

Harvey Weinstein's 2020 conviction on rape charges has been overturned by the State of New York Court of Appeals, which ordered a new trial. Weinstein will remain behind bars since he was convicted in a separate case in California.

The Francis Scott Key Bridge in Baltimore collapsed early Tuesday, March 26 after a column was struck by a container ship that reportedly lost power, sending vehicles and people into the Patapsco River.

The Academy Awards will hand out filmmaking's top honor in 23 categories during the 2024 Oscars at the Dolby Theatre in Hollywood, starting at 7 p.m. Eastern, 4 p.m. PDT.

Shots from the PGA's Genesis Invitational golf tournament held at the Riviera Golf Club in the Pacific Palisades.

Even a potentially historic storm bearing down on California couldn't keep the stars away from the red carpet ahead of the 66th Grammy Awards in Los Angeles.

Who are your favorite celebrities wearing for the 2024 Golden Globe Awards? Check out our red carpet fashion photo gallery!