Torrance Lawmaker Wants Answers From FDA In Wake Of Superbug Outbreak
LOS ANGELES (CBSLA.com) — A California congressman wants answers from the Food and Drug Administration in the wake of superbug infections at a local hospital now linked to contaminated medical scopes.
John Allen, a gastroenterologist and the clinical chief of digestive diseases at Yale School of Medicine, said he witnessed the bacterial infections from the same type of intestinal medical scopes in Minnesota back in 1987, according to CNN.
Now, Rep. Ted Lieu (D-Torrance) wants to know why -- just a week ago -- the FDA issued a safety warning to doctors and hospitals nationwide about the issue of the scope nearly three decades after the first infections surfaced.
"I do not know what took the FDA so long. That's why I'm calling for a hearing and investigation," Lieu said.
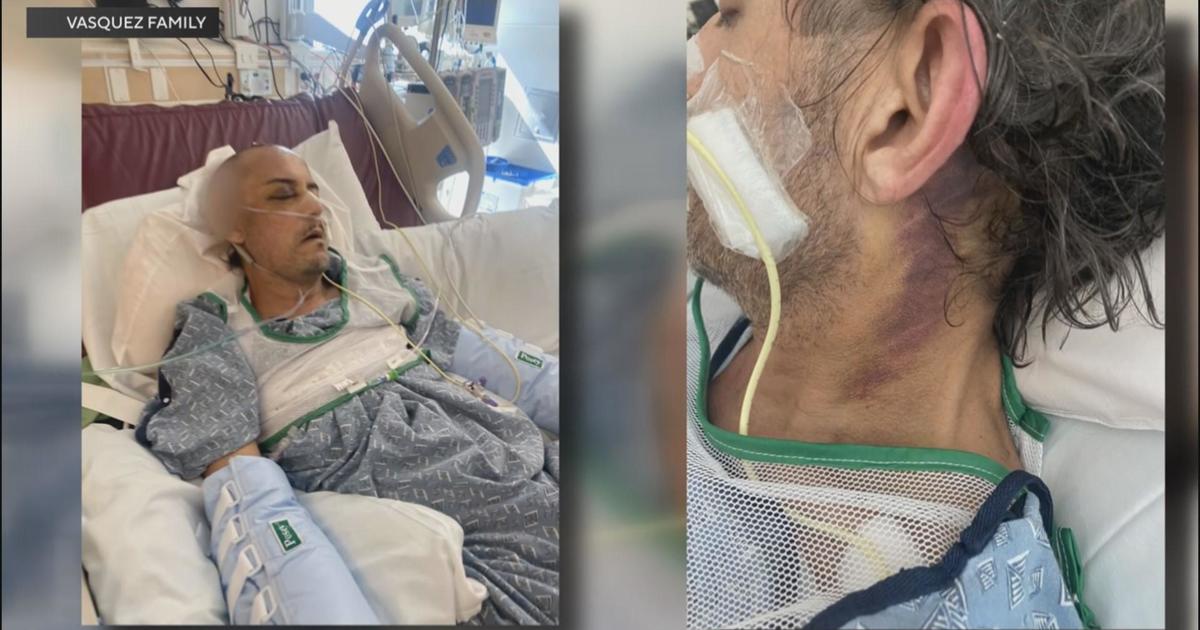
The scope at issue is called a duodeonoscope and is widely used for close-up inspection of the intestinal tract. But infections from a drug-resistant superbug traced to those medical scopes contributed to the deaths of the two patients at UCLA's Ronald Reagan Medical Center, where seven other patients are still fighting infections. Nearly 180 others may have been exposed to the bacterial infection.
As KCAL9's Dave Bryan reports, some experts believe the problem could have been addressed a long time ago.
Lawrence Muscarella, a medical device safety expert, says for years now he has been warning about the infection danger of the scopes.
"I'm frustrated because had others listened to me or had we had meetings in Washington and all of us gotten together, we could have prevented injuries. We could have saved lives," Muscarella said.
The duodeonoscopes cost about $30,000 and are used in more than 500,000 procedures per year in the U.S. By all accounts, lives are saved due to diagnostic procedures from the use of the scope.
However, the scopes are extremely difficult to completely clean and disinfect.
The manufacturer's instructions, according to an attorney who's now suing the company on behalf of some of the victims, run to more than 40 pages.
Muscarella says the infections have been traced to design problems with the scopes which could have been addressed years ago if the first cases in 1987, which did not involve a "superbug," but were non-life threatening bacterial infections, had been followed up on with action.
"Certainly, if we had started 30 years ago coming up possibly with better designs or more enhanced designs that would better facilitate the more thorough cleaning and disinfection of endoscopes, certainly, we would have been able to prevent the significant deaths that we've been seeing," Muscarella said.
Lieu said the FDA didn't issue its safety alert until after the Los Angeles Times exposed the medical scope problems last week. The agency, he says, has to be held accountable.
"I want to know what did the agency know? What are they doing about it? And what are they going to do to prevent future occurrences," he said.
As Bryan reports, the 1987 infections were not the only warning about possible trouble with the medical scopes. Muscarella warned in a medical journal in 2010 that the intestinal scoping devices had cleaning problems. In 2013, an Illinois hospital had to destroy one of the scopes when it discovered a superbug could not be entirely removed from the device.